The fast & widespread disease ‘Rapid Ohia Death (ROD)’ on the Hawaiian Islands is not slowing down. It has been found on Hawai’i Island, Kauai, Maui, and Oahu. With Ōhiʻa making up almost 80% of Hawaii’s native forests, this fungal disease may take down a broad range of the Hawaiian forests. Check out the data and results our research and development team has gathered from their trials on ROD in Hawaii.
What is it?
Ōhiʻa lehua (Metrosideros polymorpha), is an endemic, pioneering tree species. Ōhiʻa-dominated forests cover close to one million acres of land across the State of Hawai’i. Ōhiʻa can be found at elevations from sea level to greater than 9,000 ft. They are often among the first plants to colonize fresh lava substrate and are therefore instrumental in the process of soil development and ecological succession.
A new disease of Ōhiʻa was discovered in 2013. Rapid Ōhiʻa Death is a disease caused by two fungal pathogens. Ceratocystis lukuohia (destroyer of Ōhiʻa) is the name of the more aggressive fungus and Ceratocystis huliohia (disruptor of Ōhiʻa) is the less aggressive fungus. Both of these fungal pathogens can kill Ōhiʻa. The disease causes the crowns of ohia trees to turn yellow, then brown and die within days to weeks. The fungus also causes dark, nearly black, staining in the sapwood along the outer margin of trunks.

Potential Treatment
ROD fungi are in the same genus as fungi (Ceratocystis fagacearum) that cause oak wilt on the mainland. Until recently, the only effective treatment available for protecting high-value oaks is high volume treatments of the systemic fungicide propiconazole (Alamo®/Propizol®) diluted in high volume of water and injected into the lower stem or root flare of trees (Appel and Kurdyla 1992, Appel 1995). Applications of propiconazole have been made almost exclusively using macro injection systems to deliver 20ml Alamo diluted in 1-liter water per inch tree DBH. The intent is to saturate the xylem tissue of the root collar with fungicide to prevent movement of the pathogen into the above ground area of the trees. The treatment is often effective in preventing tree death for 2 years (Blaedow et al. 2010), but applications are very labor intensive. Recently, applications of Propizol in a 1:1 mix with water using Arborjet’s TREE I.V. was found to perform equally to macro-infusions of Alamo at the same active ingredient rate. It is of interest to know whether the two fungicide formulations (both Syngenta products) applied at the same rates but different methodology are effective in preventing/reducing C. lukuohia fungal infection spread within the Ōhiʻa host.
Field Testing
The following evaluation of fungicides for protection against ROD were conducted in 2018 and 2019 by Dr. Don Grosman – Technology Advancement Manager at Arborjet, in cooperation with Dr. Jennifer Juzwik – Research Plant Pathologist with the USDA Forest Service, and Dr. Lisa Keith – Research Plant Pathologist with the USDA Agriculture Research Service.
Eight (8) treatments were evaluated:
- Propizol (Syngenta) – 14.3% propiconazole applied at 20 ml in 20ml water per inch diameter. Using QUIK-jet AIR (QJA) at 100 psi + fungi (Cl)
- Alamo (Syngenta) – 14.3% propiconazole applied at 20 ml in 300 ml water per inch diameter using macro-infusion + fungi (Cl)
- Experimental fungicide formulation (Syngenta) – applied at 10 ml undiluted per inch diameter using QJA at 100 psi + fungi (Cl)
- Propizol + water – applied at 20 ml + 20 ml per inch diameter by QJA – no fungi
- Alamo + water – applied at 20 ml + 300 ml per inch diameter by Macro – no fungi
- Experimental fungicide formulation (Syngenta) undiluted 10 ml per inch diameter – no fungi
- Untreated control + fungi (Cl)
- Water Controls – applied at 20 ml per inch diameter by QJA – no fungi; & applied at 300 ml per inch diameter by Macro – no fungi
The study was conducted off the Cattle Hunt Road, Hilo Watershed Forest Reserve. Non-symptomatic Ōhiʻa test trees (29), were selected; less than 30 feet from the nearest symptomatic tree. In mid-May 2018, 2 – 6 trees were injected with one of 8 treatments at suggested rates using the Quikjet Air or Macro-infusion system. An additional 2 trees were monitored as untreated checks.
The injected trees were allowed 5 weeks to translocate chemicals prior to being challenged by inoculation of trees with Ceratocystis lukuohia spore supension. Trees were evaluated for Ōhiʻa wilt symptoms monthly for 16 months (so far).
Results
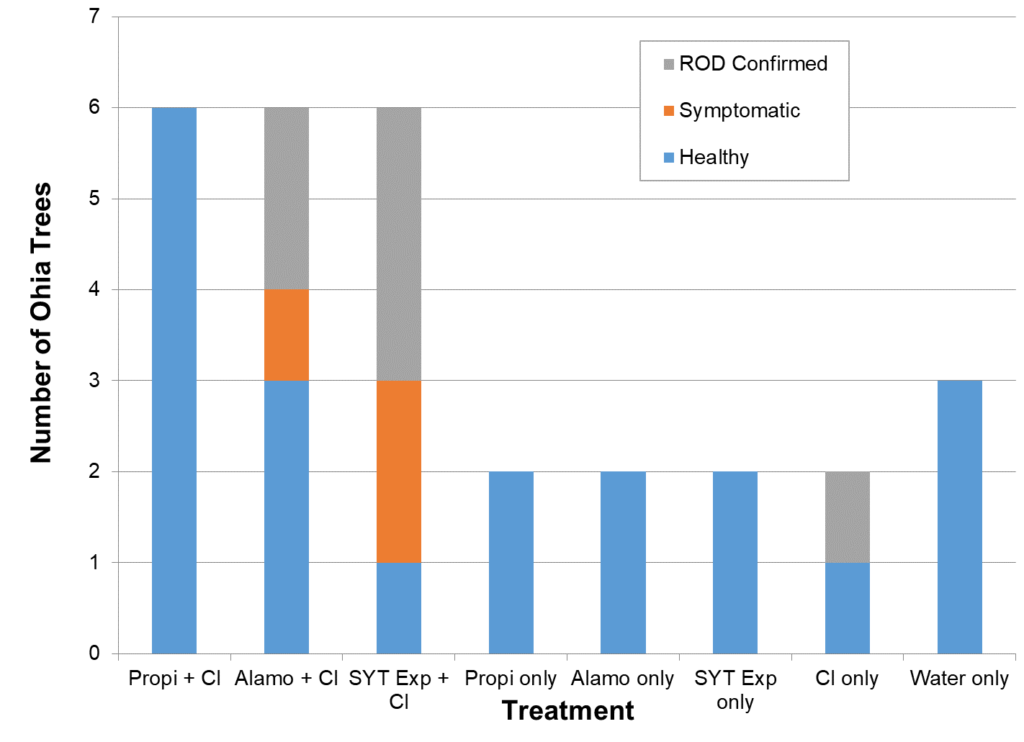
All product (propizol and Syngent Experimental) volume was fully injected into trees with the QUIK-Jet AIR system. However, application of the Alamo treatment using the macro injection system proved difficult so only a portion of the recommend volume was applied to the six trees.
All trunk-injected Ōhiʻa trees were asymptomatic 2 month (July 2018) after trial initiation, indicating no natural or inoculation success. The two untreated trees that were inoculated did appear to show some Ōhiʻa wilt symptoms, but infection was not confirmed at that time.
By 16 months post inoculation (end of October 2019) only one tree with fungi inoculation alone (Cl only), five (5) trees with Syngenta Experimental injection combined with fungi (SYT Exp. + Cl) and three (3) with Alamo with fungi (Alamo = CI) had expressed some Ōhiʻa wilt symptoms (Fig. 1). Of these, six were confirmed to have the pathogenic fungi present in upper crown. The data indicates that higher rates of propiconazole (Propizol) are effective in preventing the movement and expression of the fungi. We intend to continue monitoring study trees through 2020.
References:
Appel, D.N. 1995a. The oak wilt enigma: perspectives from the Texas epidemic. Ann. Rev. of Phytopathology. 33: 103-118.
Appel, D.N. 1995b. Chemical control of oak wilt. In: Appel, D.N. and R.F. Billings (eds.) The Proceedings of the National Oak Wilt Symposium. Information Development Inc., Houston, TX pp. 81-88.
Blaedow, R.A., J. Juzwik and B. Barber. 2010. Propiconazole distribution and effects on Ceratocystis fagacearum survival in roots of treated red oaks. Phytopathology 100: 979-985.
Camilli, K., D.N. Appel, and W.T. Watson. 2009. Studies on pruning cuts and wound dressings for oak wilt control, pp. 115-128. In R.F. Billings and D. N. Appel (eds.). Proceedings National Oak Wilt Symposium, June 4-7, 2007, Austin, TX. Texas Forest Service Publ. 166.
Koch, K.A., G.L. Quiram, and R.C. Venette. 2010. A review of oak wilt management: A summary of treatment options. Urban Forestry & Urban Greening. 9: 1-8.
Mayfield III, A.E., E.L. Benard, J.A. Smith, S.C. Bernick, J.M. Eickwort, and T.J. Dreaden. 2008. Effect of propiconazole on laurel wilt disease development in redbay trees and on the pathogen in vitro. Arboriculture & Urban Forestry. 34: 317-324.
Peacock, K.L. and D.W. Fulbright. 2009. Effective longevity of propiconazole following injection into Quercus rubra, pp. 175-184. In R.F. Billings and D. N. Appel (eds.). Proceedings National Oak Wilt Symposium, June 4-7, 2007, Austin, TX. Texas Forest Service Publ. 166.
